Clinical Outcomes Data: What Studies Show Providers About Generic Medications
 Dec, 4 2025
Dec, 4 2025
When a patient walks into your office asking why they’re switching from their brand-name blood pressure pill to a cheaper generic, what do you say? You know the science says they’re the same. But you’ve also heard patients say, “The generic doesn’t work like the original.” So where does the truth lie? The answer isn’t in anecdotes-it’s in decades of rigorous clinical outcomes data.
Generics Aren’t Just Cheaper-They’re Clinically Equivalent
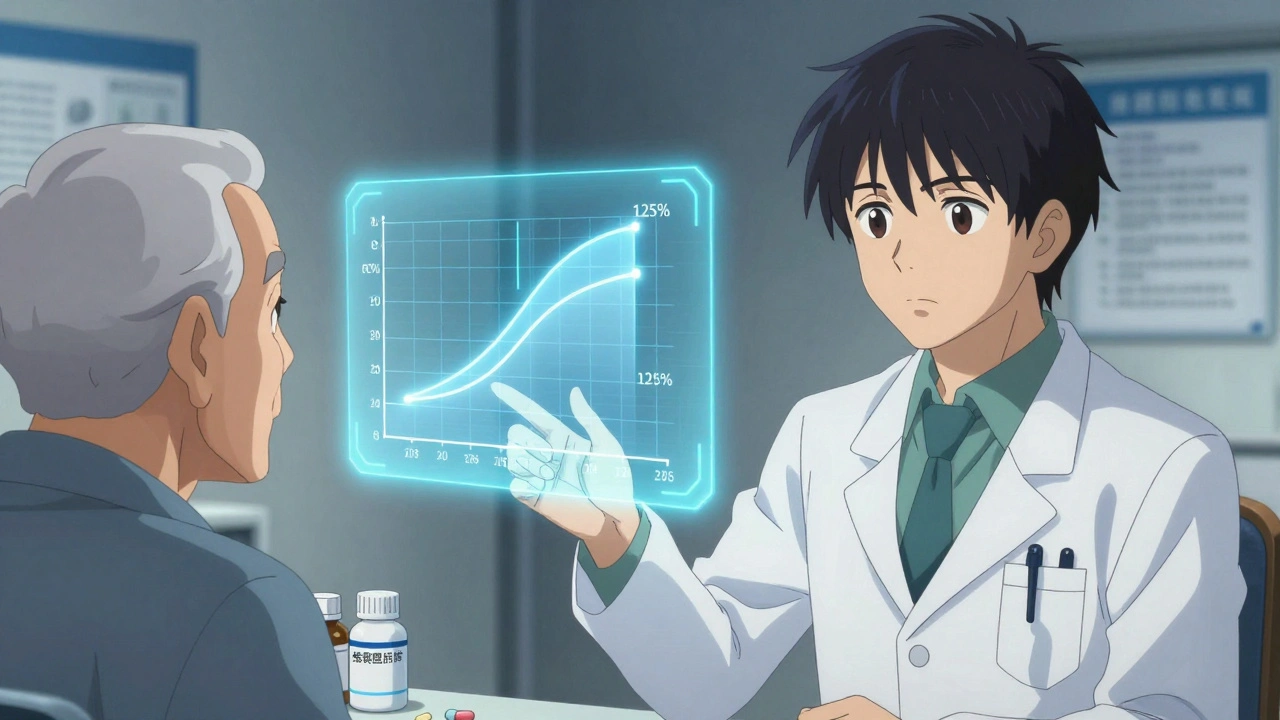
The idea that generic drugs are inferior is a myth that won’t die. But the data doesn’t support it. Since the 1984 Hatch-Waxman Act, the FDA has required generics to meet strict standards: same active ingredient, same strength, same dosage form, and-most importantly-same bioequivalence. That means the body absorbs the drug at nearly identical rates and levels as the brand-name version. The acceptable range? 80% to 125% of the brand’s pharmacokinetic profile. For most drugs, that’s not just close-it’s clinically indistinguishable. A 2019 study in PLOS Medicine analyzed over 1.3 million patient records across 14 different drug classes. The results? For 12 out of 16 comparisons, there was no statistically significant difference in outcomes between generics and brand-name drugs. For drugs like amlodipine and quinapril, generics actually showed slightly better results in preventing heart attacks and strokes. And for alendronate (used for osteoporosis) and glipizide (for diabetes), the fracture and insulin initiation rates were identical. Even in high-stakes areas like cardiovascular disease, the evidence is clear. A 2008 meta-analysis of 47 studies found that 89% showed no difference in effectiveness or safety between generic and brand-name heart medications. The remaining 11% showed mixed results-but never a consistent advantage for the brand.What About Psychiatric Drugs? The Nuance Matters
This is where things get trickier. Some studies show small increases in psychiatric hospitalizations when patients switch from brand-name escitalopram or sertraline to generics. But here’s the catch: the same pattern showed up when researchers compared authorized generics-identical to the brand, just sold under a different label-to the original brand. That suggests it’s not the drug itself. It’s perception. Patients who’ve been on a brand-name antidepressant for years may believe the blue pill works better than the white one-even if they’re chemically identical. That belief can influence how they report side effects, how likely they are to stick with the medication, and even how their brain responds to treatment. The drug doesn’t change. But the patient’s experience might. The FDA’s own 2017 review found no consistent evidence that generic psychiatric drugs cause worse outcomes. And when they tracked whether patients switched back to the brand after starting a generic, the rate was the same whether they got a traditional generic or an authorized one. If the drug were truly less effective, you’d see more dropouts. You don’t.Why Do Some Patients Switch Back? It’s Not the Medicine
You’ve seen it: a patient comes in frustrated. “The generic made me feel weird.” You check the label. Same active ingredient. Same dose. Same manufacturer, even. But the pill looks different. Smaller. Different color. Maybe even a different shape. That’s the real issue-not efficacy, but appearance. Inactive ingredients like dyes and fillers change between brands and generics. For most people, it’s harmless. But for some, especially those on long-term medications, the visual change triggers anxiety. A 2019 FDA study confirmed: these cosmetic differences don’t affect clinical outcomes. Yet they’re the #1 reason patients report “the generic isn’t working.” The data also shows that patients on generics are no more likely to stop their medication than those on brand-name drugs. In fact, the rate of discontinuation was nearly identical. The only exception? Authorized generics-those made by the brand company but sold as generics-had slightly higher emergency room visit rates. But again, no difference in actual clinical outcomes. This points to one thing: patient expectations matter more than pharmacology.
When Should You Be Cautious? The 3% Rule
Not all generics are created equal-technically. The FDA’s Orange Book classifies drugs as “A-rated” (therapeutically equivalent) or “B-rated” (not equivalent). Over 97% of generics are A-rated. The remaining 3%? These are narrow therapeutic index (NTI) drugs-medications where tiny differences in blood levels can cause big problems. Think: warfarin, levothyroxine, tacrolimus, phenytoin. For these, bioequivalence standards are tighter. Some require scaled bioequivalence testing, which accounts for how much the drug’s levels vary within a single person over time. A 2020 study in Nature Scientific Reports followed transplant patients switching between brand and generic tacrolimus over 42 days. No significant differences in rejection rates or blood levels. Still, for NTI drugs, many providers prefer to stick with one manufacturer-whether brand or generic-to minimize variability. It’s not because generics are unsafe. It’s because consistency reduces risk. If a patient does well on a specific generic, keep them on it. Don’t switch to another generic unless necessary.Cost Savings Are Real-And Life-Changing
Let’s talk numbers. In 2021, generic drugs saved the U.S. healthcare system $377 billion. Since 2009, that total exceeds $2.2 trillion. The average generic costs 80-85% less than the brand. For a patient on multiple chronic medications, that’s hundreds-sometimes thousands-of dollars a year. A 2020 study of 3.5 million Medicare beneficiaries found that patients on generics had higher five-year survival rates in unadjusted data. But when researchers adjusted for health status-because healthier people are more likely to be prescribed generics-the gap closed. That’s not because generics are better. It’s because patients on generics are often those who can’t afford the brand. The real takeaway? Access to affordable medication saves lives. The Congressional Budget Office estimates generics will save $158 billion annually through 2027. That’s not just a line item on a budget. That’s a diabetic who can afford insulin. A heart patient who doesn’t skip doses because of cost. A senior who doesn’t have to choose between meds and groceries.
What the Evidence Means for Your Practice
Here’s what you need to do:- Prescribe generics by default. Unless there’s a clear clinical reason not to, choose the A-rated generic. The American College of Physicians says it plainly: “Prescribe generics when available.”
- Don’t assume patient complaints mean the drug doesn’t work. Ask: “Is it the pill’s appearance? The cost? The side effects?” Address the real issue.
- For NTI drugs, be consistent. If a patient is stable on a specific generic, don’t switch unless necessary. Document the product used.
- Educate patients. Tell them the FDA requires generics to meet the same standards as brand-name drugs. The average review time for a generic? 10 months. That’s not rushed. That’s rigorous.
- Use authorized generics when possible. These are made by the brand company. They’re identical to the original-just cheaper. Patients often accept them more readily.
Bottom Line: The Science Is Settled
The data doesn’t lie. For nearly every drug class-cardiovascular, metabolic, neurological, even psychiatric-generics deliver the same outcomes as brand-name drugs. The differences you hear about? They’re not in the pills. They’re in the perception, the cost, the appearance, the fear of change. You’re not just prescribing medicine. You’re prescribing access. And the evidence shows that generics don’t just save money-they save lives.Are generic drugs really as effective as brand-name drugs?
Yes. Over 90% of prescriptions in the U.S. are filled with generics, and decades of clinical studies confirm they work the same way as brand-name drugs. The FDA requires generics to have the same active ingredient, strength, and bioequivalence-meaning the body absorbs them at nearly identical rates. Large studies involving millions of patients show no meaningful difference in outcomes for most medications.
Why do some patients say generics don’t work for them?
Often, it’s not the drug-it’s the change. Generics can look different in color, shape, or size due to different inactive ingredients. For patients on long-term meds, even a small visual change can trigger anxiety or belief that the new pill isn’t as strong. Studies show these concerns don’t correlate with actual differences in effectiveness. Addressing patient fears with clear education often resolves the issue.
Are there any drugs where generics aren’t safe to use?
For the vast majority of drugs, yes-generics are safe. But for a small group called narrow therapeutic index (NTI) drugs-like warfarin, levothyroxine, or tacrolimus-tiny changes in blood levels can matter. The FDA classifies these as “B-rated” in the Orange Book. While most NTI generics are still safe and effective, many providers prefer to keep patients on the same formulation-brand or generic-to avoid variability. Always check the Orange Book for therapeutic equivalence ratings.
Do generics cause more side effects?
No. FDA data from 2015-2020 shows only 0.02% of all adverse drug reports involved generic-specific concerns, compared to 3.2% for brand-name drugs. Most side effects are due to the active ingredient, not the manufacturer. If a patient reports new side effects after switching, investigate other causes first-like dosage changes, interactions, or psychological factors.
What’s the difference between a generic and an authorized generic?
An authorized generic is made by the original brand-name manufacturer but sold under a different label as a generic. It’s chemically identical to the brand-same ingredients, same factory, same packaging. The only difference? Price. Authorized generics often have higher patient acceptance because they look and feel the same as the brand, without the cost. They’re a great option when patients resist switching.
Should I always prescribe the cheapest generic?
Not necessarily. While cost matters, consistency matters more-especially for chronic conditions. If a patient is stable on a particular generic, switching to a cheaper version from a different manufacturer could introduce variability, even if both are FDA-approved. For NTI drugs, stick with the same formulation. For others, choose based on patient history, cost, and tolerance-not just price.

Marvin Gordon
December 4, 2025 AT 15:56Love this breakdown. So many providers still cling to outdated myths about generics. The data is crystal clear - it’s not the drug, it’s the fear. Patients need education, not assumptions. Keep pushing this message.
Stephanie Bodde
December 5, 2025 AT 19:50This is so important!! 🙌 I’ve seen patients cry because they think the ‘blue pill’ is ‘weaker’ than the ‘white one’ - same exact chemistry. A little empathy and a printed FDA fact sheet goes a LONG way. Thank you for writing this.
Rupa DasGupta
December 7, 2025 AT 08:56Okay but have you ever seen what happens when someone switches from brand Lexapro to generic escitalopram and suddenly they’re sobbing in the grocery store? 😭 It’s not ‘perception’ - it’s a chemical shift. The body remembers. And no, I’m not crazy. I’ve been there.
Annie Grajewski
December 9, 2025 AT 03:44lol so the FDA says its all the same but then why do i keep hearing about people getting seizures from generic phenytoin? oh wait because they switched between 3 different generics in 2 weeks and no one tracked it. classic. also who wrote this? a pharma rep? 🤔
sean whitfield
December 10, 2025 AT 10:26Generics are just the government’s way of making you take the placebo version of your medicine while the rich get the real stuff. The FDA is just a front for Big Pharma. You think they’d let a $2 pill compete with a $200 one? Come on. Wake up.
Carole Nkosi
December 11, 2025 AT 06:22Let me be brutally honest - this is performative medicine. You’re not saving lives with generics. You’re saving the system’s budget while patients suffer silently because they can’t afford to complain. The ‘data’ ignores the lived reality of withdrawal, anxiety, and invisible suffering. You’re not a doctor. You’re a cost-center.
William Chin
December 12, 2025 AT 03:19As a clinical pharmacologist with 22 years in the field, I must emphasize that the bioequivalence thresholds of 80-125% are statistically acceptable but clinically perilous for certain populations - particularly geriatric, renal-impaired, and polypharmacy patients. The assumption that ‘same active ingredient = same outcome’ is dangerously reductive. I have personally documented cases of therapeutic failure following generic substitution in NTI drugs where the manufacturer’s dissolution profile varied by 18% - within FDA limits, but outside physiological tolerance. This is not a debate about cost. It is a debate about precision medicine.
Manish Shankar
December 13, 2025 AT 04:32Thank you for this comprehensive and scientifically rigorous analysis. In India, where access to affordable medication is a daily struggle for millions, the evidence supporting generic equivalency is not merely academic - it is life-saving. I have witnessed patients with hypertension and diabetes survive only because generics allowed uninterrupted therapy. The psychological barriers you describe are real, but they are addressable through structured counseling. We must not allow misinformation to undermine public health gains.
Philip Kristy Wijaya
December 13, 2025 AT 19:13People think the pill looks different so it must be weaker - but they don’t realize the brand-name version they’ve been on for 10 years is itself a generic manufactured by Teva or Mylan under a different label. The entire brand-name illusion is a marketing construct. The real villain isn’t the generic. It’s the pharmaceutical industry’s decades-long campaign to make patients fear anything that doesn’t come in a branded bottle with a logo
Michael Dioso
December 14, 2025 AT 12:51Oh wow another one of these posts. You know what’s really happening? The FDA doesn’t test the actual pills. They test the powder in a lab. So if your generic has 10% less active ingredient because the factory didn’t mix it right - too bad. You’re the guinea pig. And don’t even get me started on the fact that most generics are made in China and India. You think they care if your blood pressure spikes? Nah. They care about the 80% bioequivalence loophole. This isn’t science. It’s capitalism with a white coat.
Jimmy Jude
December 15, 2025 AT 21:57Let’s be real - this is the same energy as when people said ‘vaccines don’t work, it’s just herd immunity’. You’re not saving lives. You’re saving money. And if your patient dies because they switched to a generic and had a stroke? Congrats. You’re a hero of cost-efficiency. But I’m not buying it. The body knows. The soul knows. The placebo effect isn’t placebo when it’s the only thing keeping someone alive.
ashlie perry
December 16, 2025 AT 07:38so like… the FDA says generics are fine but then why does my friend’s mom have to go to the er every time she switches? and why does the brand name one make her feel like she has a heartbeat again but the generic makes her feel like a zombie? its not in my head. its in my body. and you cant prove me wrong with charts.
Mark Ziegenbein
December 18, 2025 AT 01:43Here’s the uncomfortable truth nobody wants to admit - the entire generic drug system is a Ponzi scheme built on the backs of patients who can’t afford to fight back. The FDA approves generics based on statistical averages - not individual biology. One person’s 120% bioequivalence is another person’s death sentence. And the medical establishment? They’re too busy sipping their $12 coffee and patting themselves on the back for ‘saving money’ to notice that the patient who stopped taking their meds because they felt ‘off’… never came back. Because they didn’t die. They just disappeared. Into the shadows of untreated illness. And you call that progress?