International Drug Safety Monitoring Systems Explained
 Dec, 19 2025
Dec, 19 2025
Every time someone takes a medicine, there’s a quiet system working behind the scenes to make sure it’s still safe. This isn’t science fiction-it’s pharmacovigilance, the global network that tracks harmful side effects of drugs after they’ve been approved and sold to millions. Without it, we wouldn’t know about dangers like thalidomide’s birth defects in the 1960s, or the increased risk of dengue hemorrhagic fever linked to the Dengvaxia vaccine in the Philippines. Today, this system is more complex, more connected, and more critical than ever.
How the Global Drug Safety Network Works
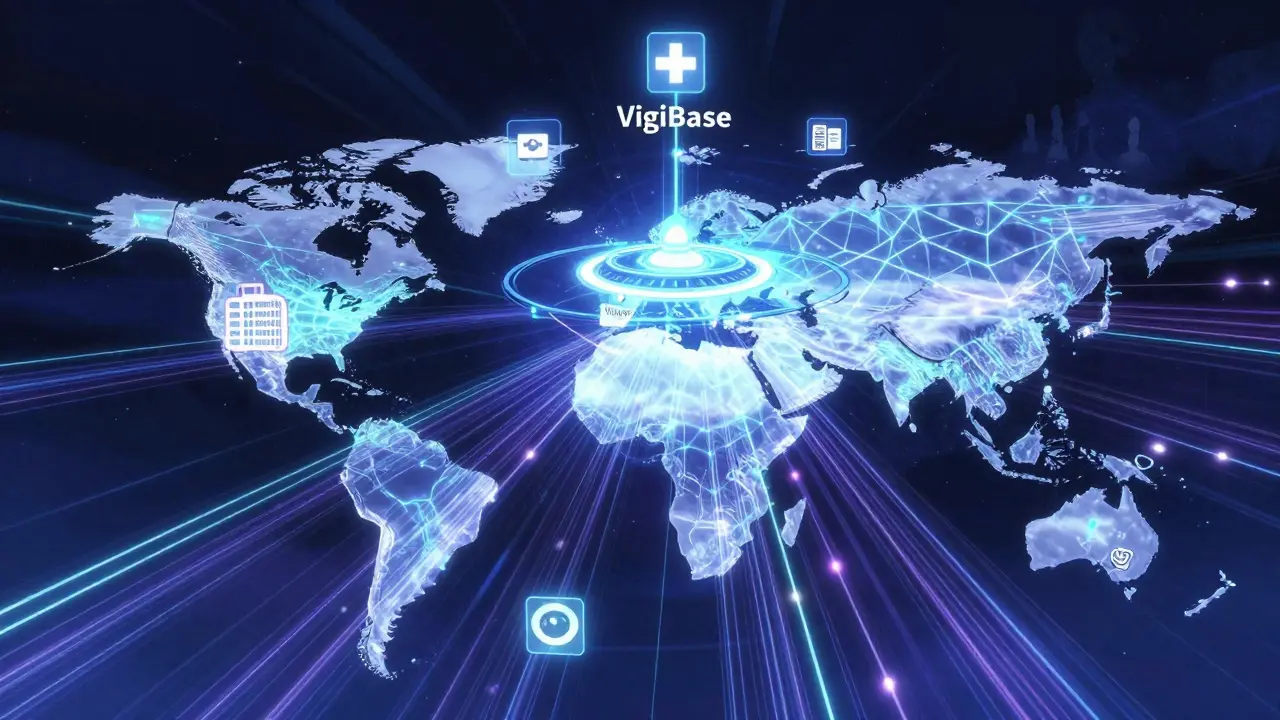
The backbone of international drug safety monitoring is the WHO Programme for International Drug Monitoring (PIDM), launched in 1968 after a 1963 resolution by the World Health Assembly. It’s not a single agency. It’s a network of 170+ countries, each with its own national center that collects reports of adverse drug reactions from doctors, pharmacists, patients, and hospitals.
These reports-called Individual Case Safety Reports (ICSRs)-are sent to the Uppsala Monitoring Centre (UMC) in Sweden, which runs the global database called VigiBase. As of 2023, VigiBase holds over 35 million reports, up from just 5 million in 2012. That’s a 700% increase in just over a decade. Each report includes details like the drug name, the patient’s symptoms, age, gender, and when the reaction happened.
To make sense of this massive amount of data, standardized tools are used. Drugs are coded using WHODrug Global, a dictionary with over 300,000 medicine names. Symptoms are classified using MedDRA, a medical terminology system with 78,000+ terms. Reports are transmitted electronically using the E2B(R3) standard, ensuring consistency across borders.
Regional Systems: EU, U.S., and Beyond
Not all countries operate the same way. The European Union has its own system, EudraVigilance, which handles about 1.2 million new reports every year. Unlike the WHO’s voluntary network, the EU system is legally binding. Pharmaceutical companies must report adverse events within 15 days of becoming aware of them. The European Medicines Agency’s Pharmacovigilance Risk Assessment Committee (PRAC) reviews signals and can recommend changes to drug labels or even withdrawals.
The U.S. runs the FDA Adverse Event Reporting System (FAERS), which gets around 2 million reports annually. While FAERS doesn’t feed directly into VigiBase, the U.S. does contribute data to it. The key difference? The FDA doesn’t require manufacturers to report within a strict deadline, and the system relies heavily on voluntary submissions from healthcare providers and patients.
Meanwhile, countries like the UK have advanced their own systems. The Yellow Card Scheme allows healthcare professionals to report side effects via a mobile app. In 2022, 78% of reports came through digital channels, and 95% were submitted within 48 hours. That’s fast. But most countries don’t have that kind of infrastructure.
The Big Gap: Rich Countries vs. Poor Countries
Here’s the uncomfortable truth: the global system is lopsided. High-income countries, which make up only 16% of the world’s population, submit 85% of all reports to VigiBase. Sweden, for example, reports 1,200 adverse events per 100,000 people each year. Nigeria? Just 2.3 per 100,000. That doesn’t mean Nigerians don’t have side effects-it means they’re not being recorded.
Why? Money. A WHO assessment found that low-income African nations spend an average of $0.02 per person annually on pharmacovigilance. High-income countries spend $1.20-60 times more. Many clinics in low-resource settings don’t have internet, computers, or trained staff to file reports. In Ethiopia, after implementing a digital tool called PViMS, reporting time dropped from 90 days to 14. But even then, only 35% of health facilities submit reports regularly, mostly because of poor connectivity.
Training is another issue. WHO recommends 40 hours of specialized training for pharmacovigilance officers. A 2022 survey in Southeast Asia found 68% had received less than 15 hours. Without proper training, reports are incomplete, inaccurate, or never made at all.

How Technology Is Changing the Game
Artificial intelligence is now helping sift through millions of reports to find hidden patterns. UMC’s AI system, introduced in 2023, cut false positive signals by 28% compared to older methods. That means fewer distractions and faster detection of real dangers.
Electronic reporting tools are spreading. Since 2020, 45 low- and middle-income countries have adopted digital systems for vaccine safety monitoring. Transmission time for vaccine-related reports dropped from 60 days to 7. That’s life-saving speed when a new outbreak or unusual reaction emerges.
Soon, a new global standard called ISO IDMP will roll out. It will standardize how medicines are identified across 100+ data points-like active ingredients, dosage forms, and manufacturers. Right now, a drug called "ibuprofen" might be listed differently in 10 countries. With IDMP, systems can match reports accurately across borders, improving signal detection by up to 40%.
Who’s Responsible? And Who’s Being Left Behind?
The pharmaceutical industry is investing heavily. The global pharmacovigilance market hit $5.38 billion in 2022 and is expected to grow to $13.17 billion by 2030. The top 50 drug companies now have an average of 250 full-time staff dedicated to safety monitoring-up from 150 in 2018. They’re required to do this by law.
But national governments? Not always. Only 62% of the 135 countries assessed by WHO have basic pharmacovigilance systems in place. 28% have no formal system at all. And 32% of low-income country programs rely on donor funding. When that funding dries up, the system collapses.
There’s also a problem with consistency. A 2023 study found that EU and U.S. experts agreed on whether a drug caused a reaction in only 63% of cases. Why? Because there’s no universal method for determining causality. One country says "likely," another says "possible," and the signal gets lost in translation.
What’s Next for Drug Safety?
The system is evolving. Countries like Ukraine reactivated their national pharmacovigilance center in March 2023 after years of disruption. Zanzibar joined the WHO network in January 2024. Yemen became a member in November 2022. These aren’t just bureaucratic updates-they’re signs that even in crisis, countries are prioritizing patient safety.
Public access is improving too. Since 2015, VigiAccess has allowed anyone to search anonymized data from VigiBase. Over 12 million people have used it to look up drug safety information. Patients, researchers, and journalists now have tools to ask questions and hold systems accountable.
The goal isn’t perfection-it’s progress. The system won’t catch every side effect. Some reactions only show up after millions of people have taken a drug. But the more data we collect, the faster we spot the patterns. And the faster we spot them, the sooner we can warn people, update labels, or pull dangerous drugs off the market.
This isn’t just about regulators and scientists. It’s about you. If you’ve ever had an unexpected reaction to a medication, your report matters. It could help someone in another country avoid the same problem. You don’t need to be a doctor. You don’t need to be in a rich country. You just need to speak up.
Frequently Asked Questions
What is pharmacovigilance?
Pharmacovigilance is the science and activities focused on detecting, assessing, understanding, and preventing adverse effects or any other problems related to medicines. Its main goals are to protect patient safety and support public health by ensuring medicines remain safe throughout their use.
How does VigiBase work?
VigiBase is the global database managed by the Uppsala Monitoring Centre that collects Individual Case Safety Reports (ICSRs) from over 170 countries. Each report includes details about the drug, the patient, and the adverse reaction. Data is standardized using MedDRA and WHODrug Global, then analyzed for patterns that might indicate new safety risks.
Why do high-income countries report more adverse reactions?
They have better infrastructure: trained staff, digital reporting tools, internet access, and funding. They also have stronger public awareness campaigns and legal requirements for healthcare providers to report. It’s not that side effects happen more often-it’s that they’re more likely to be noticed and recorded.
Can patients report adverse drug reactions themselves?
Yes. In many countries, including the UK, Canada, and the U.S., patients can submit reports directly through online portals, apps, or phone lines. In the UK’s Yellow Card Scheme, patients account for over 30% of reports. Your report can help save someone else’s life.
What’s the difference between EudraVigilance and VigiBase?
EudraVigilance is the EU’s mandatory system for collecting adverse drug reaction reports from member states and pharmaceutical companies. VigiBase is the WHO’s global database that collects voluntary reports from over 170 countries. EudraVigilance has stricter deadlines and legal enforcement; VigiBase has broader global coverage but less regulatory power.
Are AI tools improving drug safety monitoring?
Yes. AI helps analyze millions of reports faster and with fewer false alarms. UMC’s AI system reduced false positive signals by 28% in 2023. This means regulators can focus on real dangers instead of noise, speeding up responses to emerging safety issues.
What is ISO IDMP and why does it matter?
ISO IDMP is a new global standard that will standardize how medicines are identified across 100+ data elements like active ingredients, dosage forms, and manufacturers. Right now, the same drug may be named differently in different countries. IDMP will make it easier to match reports across borders, improving detection of global safety signals by up to 40%.
How can low-income countries improve their drug safety systems?
They need investment in digital tools like PViMS, training for healthcare workers, and stable funding. Partnerships with international organizations, mobile-based reporting apps, and simplified reporting forms can help overcome infrastructure gaps. Even small steps-like training one pharmacist per clinic-can dramatically increase reporting rates.

Nancy Kou
December 21, 2025 AT 05:17Just read this and I’m honestly stunned at how much work goes into keeping drugs safe after they’re on the market. Most people think once it’s approved, it’s good to go - but no, it’s a living, breathing system that’s constantly watching for trouble. That 700% increase in reports over a decade? That’s not more side effects - that’s better reporting. We’re finally getting better at listening.
And the fact that patients in the UK can report via a mobile app? That’s the future. Everyone should have that access, not just people in rich countries.
My aunt had a reaction to a blood pressure med in 2019. She filed a Yellow Card. Two months later, the label got updated. That’s real impact.
Don’t underestimate your voice. Even if you think it’s ‘just one report,’ it’s data point number 35 million.
Thank you for writing this.
Hussien SLeiman
December 23, 2025 AT 01:14Let’s be real - this whole system is a corporate theater with a side of global inequality. You talk about VigiBase and AI and ISO standards like they’re magic solutions, but the truth is, Big Pharma spends billions on pharmacovigilance because they’re forced to - not because they care. They’re not fixing the problem; they’re just managing the PR fallout.
Meanwhile, Nigeria reports 2.3 adverse events per 100,000 people? That’s not because they’re safe - it’s because the system is designed to fail them. The WHO doesn’t fund these systems - they just hand out brochures and call it ‘capacity building.’
And don’t get me started on ‘patient reporting.’ Sure, you can file a report in Canada, but if you’re in rural India and your child has seizures after a vaccine, who do you call? The local clinic doesn’t have electricity, let alone a digital portal. So your child’s suffering becomes a footnote in a spreadsheet no one reads.
AI reduces false positives? Great. But what about the real positives that never get reported because the patient never made it to a doctor? This isn’t science. It’s a privileged echo chamber dressed up as global health.
And now they’re rolling out ISO IDMP? Another standard. Another cost. Another barrier for the countries that can’t afford it. Progress? Or just more paperwork for the rich to feel good about themselves?
Janelle Moore
December 24, 2025 AT 07:07Wait - so you’re telling me the government and drug companies are secretly tracking every single side effect… but they’re not telling us the real dangers? I’ve been taking statins for 7 years and my doctor never mentioned the increased risk of diabetes. And now you say they’ve got 35 million reports? Where’s the transparency? Who’s really controlling this? I bet they’re burying the worst ones. The CDC and FDA are in bed with Big Pharma - you think they’d actually pull a drug that makes billions? No way. This is all a cover-up. They want you scared of vaccines but keep selling you pills that kill slowly. Look up the ‘Vaccine Injury Compensation Program’ - they’ve paid out billions, but no one talks about it. This isn’t safety. It’s control.
Henry Marcus
December 24, 2025 AT 09:54Guillaume VanderEst
December 25, 2025 AT 11:32Okay, I’ll admit - I didn’t think twice about how drugs were monitored until I had a weird reaction to a new antibiotic. Took me three weeks to even realize it was the med. My doctor shrugged. ‘It’s rare.’ So I Googled it - found a Reddit thread from someone in Australia who had the same thing. Turns out, it was in VigiBase. Two years ago.
That’s when it hit me - I could’ve saved myself weeks of anxiety if I’d just known to report it.
So I did. Via the FDA portal. Got an auto-reply that said ‘Your report has been received.’ No follow-up. No ‘thanks.’ But I did it anyway.
Because if someone else gets this weird rash - and they’re in a country that doesn’t even have a reporting system - maybe my report helps them. Maybe it’s the one that triggers the warning.
It’s not glamorous. It’s not heroic. But it’s necessary.
So yeah. Report. Even if it feels pointless. Even if no one replies. Just do it.
Erica Vest
December 26, 2025 AT 11:02One thing missing from this article is the role of pharmacists in pharmacovigilance. In the U.S., community pharmacists are often the first to notice unusual patterns - like a sudden spike in reports of dizziness after a new generic version of a drug is released. But they’re rarely trained or incentivized to report systematically. Many don’t even know how to file an ICSR. This isn’t just a global infrastructure problem - it’s a frontline workforce problem.
Also, MedDRA and WHODrug are essential, but they’re not perfect. I’ve seen cases where a ‘headache’ was coded as a serious adverse event because the patient was a migraine sufferer and the system didn’t distinguish between baseline and new-onset. False positives aren’t just noise - they can trigger unnecessary regulatory actions that delay access to life-saving meds.
Standardization helps, but human context still matters. The system needs more nuance, not just more data.
Carolyn Benson
December 28, 2025 AT 00:16It’s funny how we treat drug safety like it’s some sacred, apolitical system. But let’s be honest - the entire framework is built on profit-driven incentives masked as public health. The EU mandates reporting? Great. But they also mandate that companies pay for the entire system. Who benefits? The companies that can afford compliance teams. The ones that can’t? They either cut corners or get pushed out of the market - which is fine, because consolidation means fewer players, more monopoly pricing.
And then there’s the ‘patient empowerment’ narrative. ‘You can report!’ they say. But if you’re undocumented, uninsured, and working two jobs just to afford your insulin, do you really have time to fill out a 12-page form in a language you barely speak? Or are you just supposed to ‘trust the system’ while your kidneys fail?
This isn’t progress. It’s capitalism with a lab coat.
And don’t even get me started on AI. You think an algorithm can understand cultural context? That a ‘fatigue’ report from a woman in rural Bangladesh means the same as one from a white man in Toronto? Of course not. The system is biased. And it’s pretending it’s not.
Chris porto
December 29, 2025 AT 04:14I think we’re missing the bigger picture here. Pharmacovigilance isn’t just about drugs - it’s about trust. Trust between patients and doctors. Trust between nations. Trust that the system won’t ignore you when you’re hurting.
When I moved from the U.S. to India for work, I was shocked how little people knew about reporting side effects. No one talked about it. No one even knew it was possible. But when I showed a local nurse how to use the Yellow Card app on my phone - she immediately started showing others. Now she’s training three clinics.
That’s the real story. Not the stats. Not the AI. Not the standards.
It’s one person, in one clinic, showing another person how to speak up.
That’s how change happens.
Not because of policy. Not because of funding.
Because someone cared enough to teach someone else how to be heard.
Aadil Munshi
December 30, 2025 AT 18:30Let’s not romanticize this. You say ‘your report matters’ - but statistically, your individual report has a 0.00003% chance of changing anything. The system is designed to drown out noise, not amplify voices. You’re not a hero for filing a report - you’re a data point in a corporate compliance dashboard.
And let’s talk about the ‘success stories’ - like the Dengvaxia case. Sure, it got pulled. But only after hundreds of kids died. And even then, the WHO didn’t act until the media exploded. The system doesn’t prevent tragedies - it reacts to them. And only when the public outcry is loud enough to make the headlines.
Meanwhile, in countries where no one’s watching, people are still getting poisoned by drugs that were pulled elsewhere - because the labels never changed. The system isn’t broken. It’s working exactly as intended: to protect profits, not people.
So yes, report if you want. But don’t pretend you’re changing the game. You’re just feeding the machine.
William Liu
December 31, 2025 AT 10:14I just wanted to say - thank you for writing this. It’s easy to feel powerless when you’re sick or confused by a side effect. But knowing that there’s a global network out there, even if it’s imperfect, gives me hope.
My sister had a rare reaction to a flu shot. We didn’t know what to do. We Googled. Found the CDC portal. Filed the report. Three months later, the vaccine insert got updated to include it.
That’s not nothing.
It’s not perfect. It’s not fast. But it’s real.
And if your report helps even one person avoid the same mistake - it’s worth it.
Keep speaking up. Even when it feels quiet. Someone’s listening.
Kinnaird Lynsey
January 2, 2026 AT 02:28Interesting how we assume more reporting = better safety. But what if we’re just creating noise? What if the real danger isn’t the drugs - it’s the overwhelming volume of low-quality reports that distract regulators from the real signals?
And yet, if we reduce reporting, we risk missing the rare but deadly reactions.
It’s a paradox.
Maybe the answer isn’t more data - it’s smarter data. Better triage. Better training for frontline providers. Maybe we need fewer reports, but higher quality ones.
And maybe we need to stop pretending this is a purely technical problem. It’s a human one. People don’t report because they’re scared, confused, or don’t trust the system.
Fix that first. The tech will follow.
Alisa Silvia Bila
January 2, 2026 AT 12:41Erica Vest
January 3, 2026 AT 10:15Just saw your comment about the rash - same thing happened to me with a new thyroid med. I reported it. Got zero follow-up. But I found out later that the same reaction was reported by three others in Canada and Germany. Three months later, the label changed. So yeah - you’re right. It matters. Even if it feels like shouting into the void. Someone, somewhere, is connecting the dots. And it’s because of people like you.