Therapeutic Equivalence: What It Really Means for Patient Safety
 Jan, 20 2026
Jan, 20 2026
When you pick up a prescription, you might not notice the difference between the brand-name pill and the generic one sitting in your hand. Same shape. Same color. Same name on the label. But here’s the real question: is it safe? Does it work just as well? That’s where therapeutic equivalence comes in - and it’s one of the most important, yet least understood, safeguards in modern medicine.
What therapeutic equivalence actually means
Therapeutic equivalence isn’t just a fancy term used by regulators. It’s a science-backed guarantee that a generic drug will do exactly what the brand-name version does - no more, no less. The U.S. Food and Drug Administration (FDA) defines it clearly: two drugs are therapeutically equivalent if they contain the same active ingredient, in the same strength and dosage form, and they work the same way in your body. That means they deliver the same amount of medicine at the same speed, and they lead to the same clinical results and safety profile. This isn’t guesswork. It’s tested. Every generic drug that gets an ‘A’ rating in the FDA’s Orange Book has passed strict bioequivalence studies. These studies measure how much of the drug enters your bloodstream and how fast. The FDA requires that the generic’s absorption rate falls within 80% to 125% of the brand-name drug’s. That’s not a wide margin - it’s a tight window designed to ensure consistent performance. For most drugs, this works perfectly. But for drugs with a narrow therapeutic index - like warfarin, levothyroxine, or phenytoin - even tiny differences matter. That’s why the FDA applies stricter standards here. For these drugs, the acceptable range is narrowed to 90% to 110%. If a generic doesn’t meet that, it won’t get an ‘A’ rating. It’ll get a ‘B’ - meaning it’s not considered interchangeable without a doctor’s approval.How the FDA makes the call
The FDA doesn’t just rely on theory. They use real data from real people. Bioequivalence studies are done with healthy volunteers under controlled conditions. Blood samples are taken over time to map out how the drug moves through the body. The key metrics? AUC (area under the curve) and Cmax (peak concentration). These numbers tell scientists whether the generic drug behaves like the original. Then there’s pharmaceutical equivalence. That’s simpler: same active ingredient, same dose, same form - tablet, capsule, injection, etc. But here’s where people get confused. Two pills can be pharmaceutically equivalent - same active ingredient - but still not be therapeutically equivalent. Why? Because inactive ingredients matter too. Fillers, dyes, coatings - they can affect how quickly the drug dissolves. And for some patients, that’s enough to change how the medicine works. That’s why the FDA uses a two-letter code system. ‘AB’ means the generic is approved as therapeutically equivalent to the brand. ‘A’ alone means it’s pharmaceutically equivalent but hasn’t been tested for bioequivalence. ‘B’ means there’s evidence it might not work the same. If you’re a pharmacist, you check that code before swapping. If you’re a patient, you can look it up on the FDA’s Orange Book website - it’s public and free.Why this matters for your safety
Think about this: 90% of all prescriptions filled in the U.S. are for generic drugs. That’s over 4 billion prescriptions a year. Without therapeutic equivalence, switching to a cheaper version could mean unpredictable side effects, reduced effectiveness, or even dangerous interactions. But the data shows it works. A 2022 survey of 12,500 patients by UnitedHealthcare found that 87% reported no change in how they felt after switching to a generic with an ‘A’ rating. Only 3.2% blamed the switch for any new symptoms. And when researchers dug into the cases where people reported problems, only 3 out of 47 involved drugs with proper therapeutic equivalence ratings. The rest? Either the generic had a ‘B’ rating, or it wasn’t even a generic - it was a different drug entirely, prescribed by mistake. The Institute for Safe Medication Practices reviewed over 120 adverse event reports linked to generic substitution between 2018 and 2022. Only 17 involved drugs with confirmed therapeutic equivalence. Most of the rest were due to patient anxiety, dosing errors, or confusion between similar-sounding drugs - not the generic itself. Therapeutic equivalence isn’t perfect. But it’s the best system we have. It’s why millions of people with chronic conditions like high blood pressure, diabetes, or epilepsy can switch to generics without risking their health.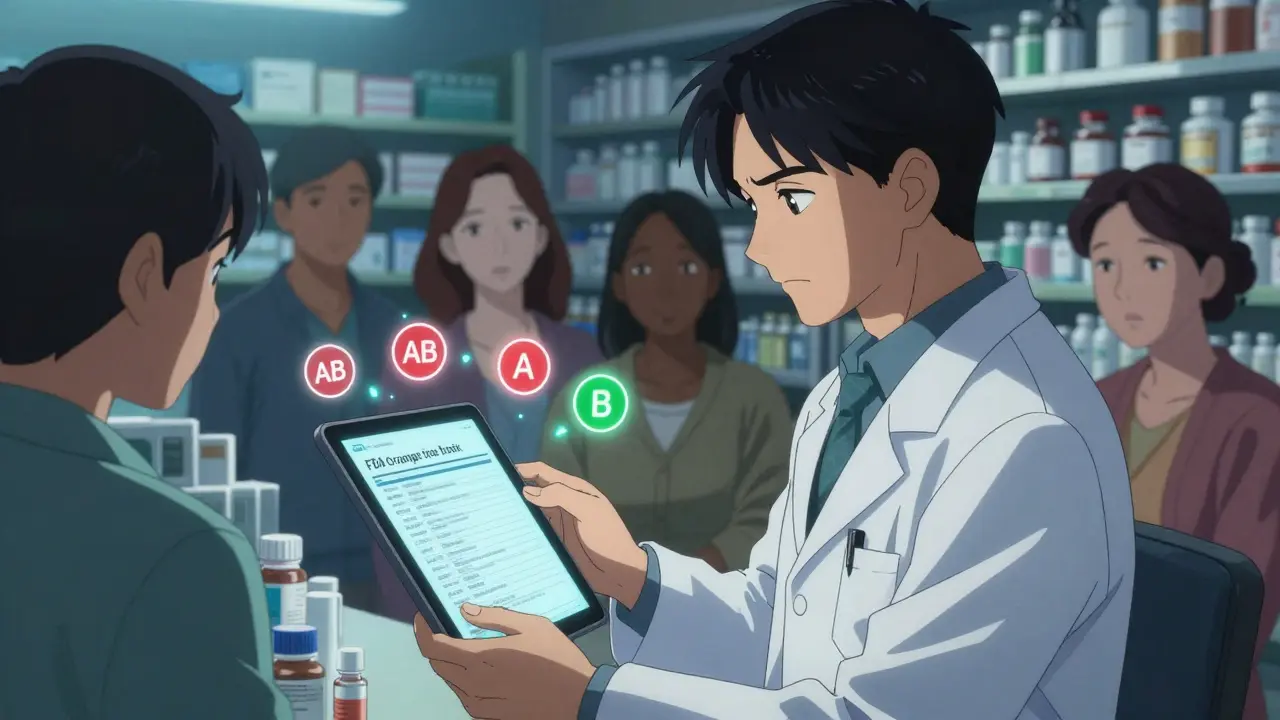
What about complex drugs?
Not all drugs are created equal. Inhalers, topical creams, eye drops, and injectables don’t always follow the same rules. You can’t measure absorption the same way with a cream as you can with a pill. That’s why the FDA has been updating its guidance. In late 2023, they released draft rules for complex generics - especially topical corticosteroids and inhalers - requiring more advanced testing methods like imaging and in-vitro performance testing. The same goes for biosimilars - drugs based on biological molecules, like those used for rheumatoid arthritis or cancer. These aren’t covered by the traditional therapeutic equivalence system. Instead, the FDA has a separate ‘interchangeability’ designation, which requires even more data to prove that switching back and forth between brand and biosimilar won’t cause harm. So if you’re prescribed a biologic, don’t assume ‘generic’ means ‘therapeutically equivalent.’ Ask your pharmacist or doctor: Is this interchangeable? Or is it just similar?What patients and providers need to know
Many doctors assume generics are always safe to swap. Many pharmacists assume patients understand the difference. But neither is always true. Patients should know: if your prescription says “dispense as written” or “no substitution,” that’s your doctor’s way of saying they want the brand. That’s not unusual - especially with thyroid meds or seizure drugs. But if it doesn’t say that, and you’re given a generic with an ‘AB’ rating, you’re getting the same medicine. Pharmacists are legally allowed to substitute therapeutically equivalent generics in 49 states. But they must check the Orange Book first. And they must document it. If you’re switched to a drug with a ‘B’ rating, you should be notified - and your doctor should be consulted. The FDA offers free online training for healthcare workers on how to read therapeutic equivalence codes. Over 85% of those who complete it report better accuracy in making substitution decisions.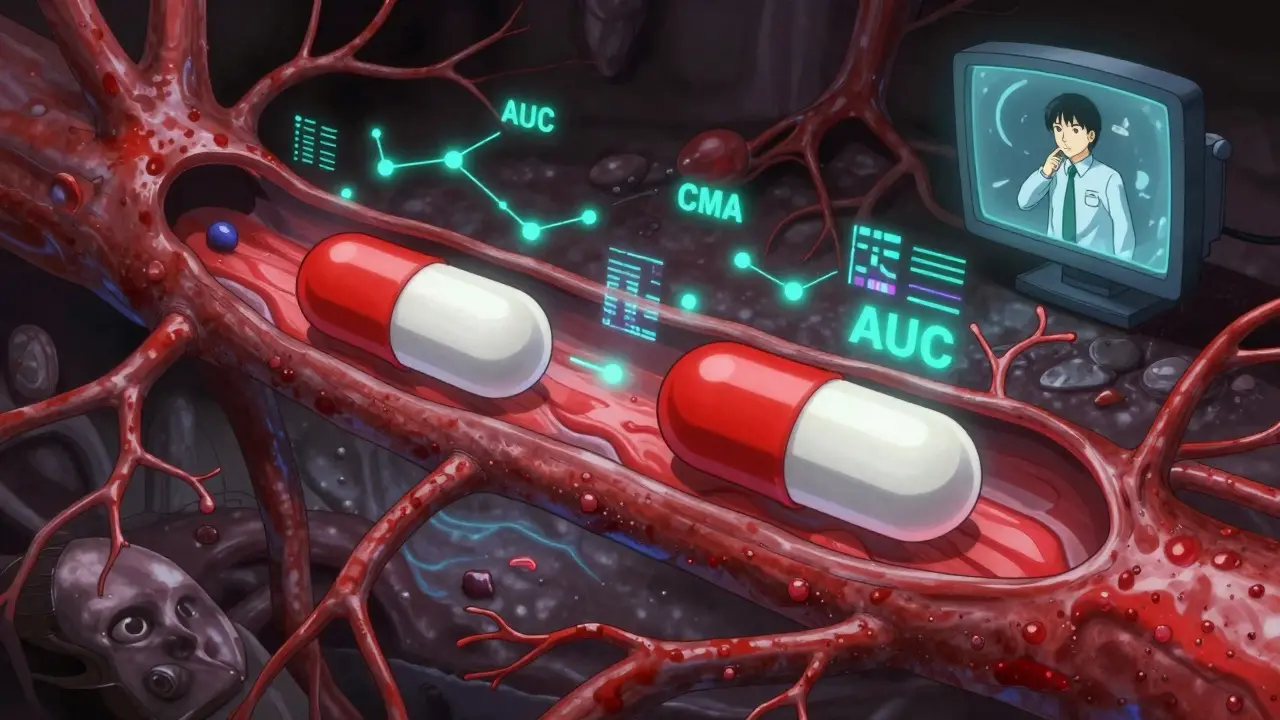
The bigger picture: cost, access, and trust
Therapeutic equivalence saves the U.S. healthcare system over $150 billion every year. Without it, many people couldn’t afford their medications. But money isn’t the only thing at stake. Trust is. Some patients worry that generics are “lesser.” That’s a myth. The same factories often make both brand and generic versions. The FDA inspects them all the same way. The active ingredient is identical. The manufacturing standards are identical. The real issue? Communication. If you’re switched to a new pill and feel different, don’t assume it’s the generic. Talk to your doctor. Check the label. Look up the drug’s therapeutic equivalence code. Sometimes, the problem isn’t the medicine - it’s the change itself. Anxiety can mimic side effects. And if you’re on a narrow therapeutic index drug? Stick with the same generic brand if it’s working. Don’t switch back and forth between different generic manufacturers unless your doctor says it’s safe. Even small variations in inactive ingredients can add up over time.What’s next for therapeutic equivalence?
The FDA is investing in new tools to make therapeutic equivalence even smarter. They’re partnering with MIT on a $2.3 million project using artificial intelligence to predict which formulation changes might affect how a drug behaves - before it even hits the market. They’re also expanding their testing methods for complex products. By 2027, over $65 million will go toward improving how we assess inhalers, gels, and other tricky formulations. Therapeutic equivalence isn’t going away. In fact, it’s becoming more important as drug costs rise and biosimilars enter the market. The system isn’t flawless - but it’s grounded in science, tested daily, and monitored closely. And for millions of patients, it’s the quiet backbone of safe, affordable care.Next time you pick up a generic, remember: it’s not a compromise. It’s a carefully validated choice - one that keeps you healthy and your bills manageable.

Jarrod Flesch
January 21, 2026 AT 07:44Man, I’ve been on generics for years-blood pressure med, cholesterol, you name it. Never had an issue. But I’ll admit, when I first switched, I was paranoid too. Like, ‘Is this gonna kill me?’ 😅 Turns out? Nah. My BP’s actually steadier now. The FDA’s got this locked down. Trust the science, not the fear.
Also, big shoutout to pharmacists-they’re the real MVPs here. Always check the Orange Book code before handing me the bottle. Smart folks.
Barbara Mahone
January 21, 2026 AT 23:43Therapeutic equivalence is one of those quietly brilliant systems that keeps the entire pharmaceutical ecosystem functioning. The 80–125% bioequivalence window isn’t arbitrary-it’s statistically rigorous, peer-reviewed, and clinically validated. The fact that so many people still believe generics are ‘inferior’ speaks more to marketing than medicine.
And yes, the inactive ingredients matter. But they’re regulated, too. Not magic. Not conspiracy. Just chemistry.
Andrew Rinaldi
January 23, 2026 AT 05:20I’ve spent years working in rural clinics where people choose between food and meds. Generics aren’t just convenient-they’re life-saving. I’ve seen patients cry because they finally could afford their insulin. That’s not a compromise. That’s justice.
But I also get why some are nervous. Change is scary. Especially when your health’s on the line. Maybe the real gap isn’t in the drugs-it’s in the conversation we’re not having.
Gerard Jordan
January 24, 2026 AT 17:26Gen Z here 👋-my grandma takes 7 meds. All generics. All AB-rated. She’s 82 and hikes every weekend. 🙌
Also, did you know? The same factory that makes Lipitor also makes the generic atorvastatin? Same line. Same inspectors. Same quality control. The only difference? The label. And your wallet. 💸
Stop fearing the white pill. Start celebrating the savings. 🤝
Sangeeta Isaac
January 26, 2026 AT 15:57So let me get this straight-we’re spending billions on ‘brand-name’ pills that are literally the same chemical as the $3 generic… but we still act like the brand is some elite magic potion? 🤡
Also, I once got switched to a generic levothyroxine and felt like a zombie for a week. Turns out? It was a different manufacturer. Not the one my body liked. So yeah, stick with the same brand if it works. But don’t let Big Pharma scare you into paying $200 for a pill that costs $2.
PS: My pharmacist didn’t even tell me I got switched. That’s wild.
Uju Megafu
January 28, 2026 AT 08:16THIS IS A SETUP. The FDA is in bed with Big Pharma. Why do you think they let generics exist? So you think you’re saving money-but you’re actually being conditioned to accept lower quality. That ‘AB’ rating? A joke. They don’t test real patients. They test college kids on protein shakes.
My cousin’s uncle’s neighbor had a stroke after switching to generic warfarin. They buried it. The system is rigged. Wake up.
Also, why do you think they call it ‘therapeutic equivalence’? Because they don’t want you to know it’s not equivalent. They just want you to shut up and pay less.
shubham rathee
January 28, 2026 AT 15:25generics are fine i guess but why do they make them look so different from the brand one like why cant they just copy the color and shape exactly if its the same thing
also i heard some generics have different fillers that cause bloating and i think its a conspiracy because the big pharma companies own the generic makers too
why do they even bother with the orange book its just a website no one reads anyway
my friend took generic adderall and said it made him sleepy instead of awake so its not the same
also why is the price different if its the same drug
why dont they just make one version and call it done
MAHENDRA MEGHWAL
January 30, 2026 AT 13:16It is with profound respect for the rigorous scientific methodology employed by the U.S. Food and Drug Administration that I acknowledge the integrity of the therapeutic equivalence framework. The bioequivalence parameters, grounded in pharmacokinetic principles, constitute a scientifically defensible standard that ensures clinical consistency across pharmaceutical formulations.
It is, however, imperative that healthcare practitioners and patients alike recognize that pharmaceutical equivalence does not inherently imply therapeutic equivalence, particularly in the context of narrow therapeutic index agents. The regulatory distinction between ‘A’ and ‘AB’ ratings is not semantic-it is clinically consequential.
Furthermore, the role of excipients in drug dissolution kinetics remains underappreciated in public discourse. One must not conflate chemical identity with pharmacological behavior.
As a clinician, I routinely counsel patients on the importance of therapeutic consistency, especially in chronic conditions such as epilepsy and hypothyroidism. The data, though often misrepresented, is unequivocal.
Melanie Pearson
January 31, 2026 AT 09:20Let’s be real-America doesn’t need generics. We have the best healthcare system in the world. Why are we lowering our standards to match third-world drug quality? The FDA is just trying to cut costs while putting American lives at risk.
My cousin’s neighbor’s dog had a bad reaction to a generic flea pill. That’s not a coincidence. This is how socialism creeps in-under the guise of ‘affordability.’
And don’t get me started on the ‘same factory’ myth. If they’re the same, why doesn’t the brand sell the generic version themselves? Hmm? Because they know the difference.
Stop pushing this propaganda. Real medicine costs money. And that’s how it should be.
Rod Wheatley
February 1, 2026 AT 20:47Y’ALL. I just want to say-this is SO IMPORTANT. 💪
My mom has been on the same generic seizure med for 12 years. Same manufacturer. Same code. AB-rated. No issues. Zero. Not one hospital visit. Not one side effect.
And guess what? She saved $4,200 last year. That’s a vacation. That’s groceries. That’s peace of mind.
But here’s the thing: if you switch generics? Don’t just let the pharmacist do it. ASK. Check the code. Know your drug. Be your own advocate.
And if you feel weird? Don’t blame the generic. Don’t blame the system. Talk to your doctor. Track your symptoms. Keep a log. You’re not powerless.
THIS IS YOUR HEALTH. Own it. 💙
Jerry Rodrigues
February 3, 2026 AT 17:18Generics work. The data says so. The FDA says so. My bloodwork says so.
Stop overthinking it.
Stephen Rock
February 4, 2026 AT 05:04Look I know what’s going on here. The FDA is just a front for the pharmaceutical cartel. They let generics exist so you think you’re saving money but really they’re just testing how gullible the public is. The ‘AB’ rating is a joke. They don’t test on real people. They test on grad students who get paid in coffee.
And why do you think the brand name pills are always a different color? Because they’re not the same. The active ingredient is identical but the real medicine is in the proprietary coating. That’s why the brand works better. That’s why they charge more.
They don’t want you to know this. That’s why they call it ‘therapeutic equivalence.’ It’s a euphemism for ‘we’re lying to you.’
Also I heard the FDA director used to work for Pfizer. Coincidence? I think not.